Since March 2010, increased growth in the 340B Program has been accompanied by increased scrutiny from state and federal governments and conflicts between various 340B Program stakeholders. A transition in U.S. Department of Health & Human Services (HHS) and Health Resources & Services Administration (HRSA) leadership may lead to changes in 340B Program policy, but the ongoing conflicts, particularly around contract pharmacies, will not likely be resolved quickly.
In this webinar, we discussed the current issues affecting 340B Program stakeholders, the tools (and their limitations) that may be employed by stakeholders and government agencies to resolve those issues, and what covered entities can expect in future developments affecting the 340B Program.
- Covered entities will likely be unable to resolve contract pharmacy issues quickly through either the current litigation or the ADR panels. While there are a number of pending cases related to the 340B Program, litigation can be inherently slow process. The Administrative Dispute Resolution (ADR) Final Rule that was published in December 2020 was recently enjoined and additional injunctions may follow. While HHS appears to be moving forward with operationalizing the ADR process, the ADR Panel members who would hear the disputes remain under review by the Biden Administration. If and when the ADR panels are finally implemented, decisions of those panels may be litigated too.
- Covered entities should review and monitor their state Medicaid program’s billing requirements for 340B drugs. State Medicaid programs must have a mechanism to identify 340B drugs when required to exclude them from [...]
Continue Reading
read more

 Subscribe
Subscribe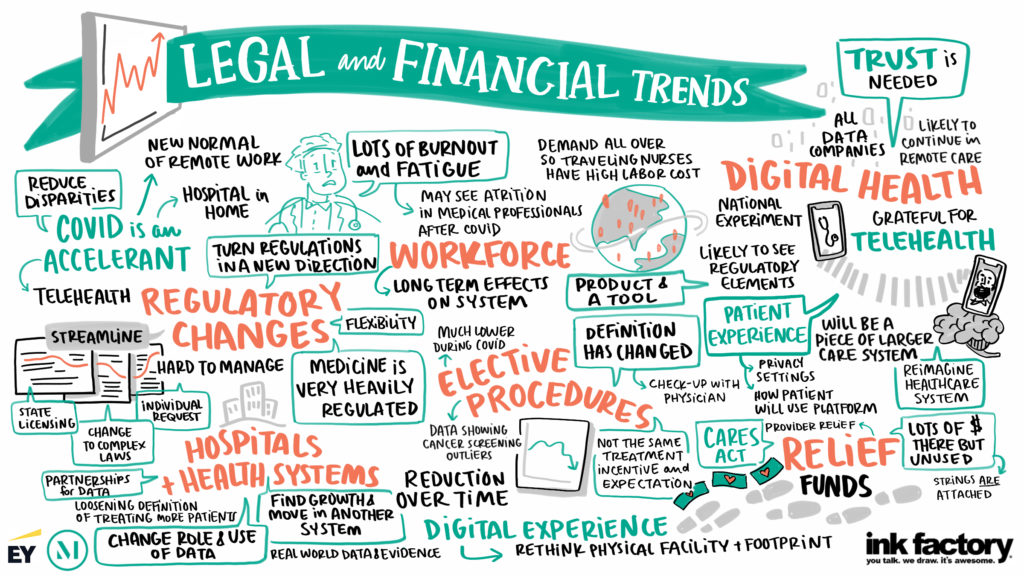

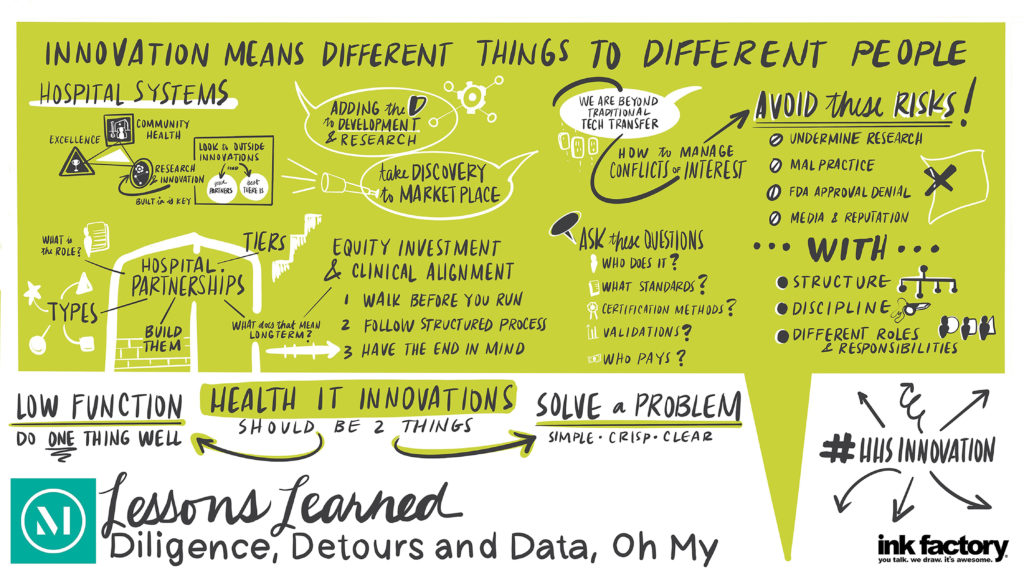
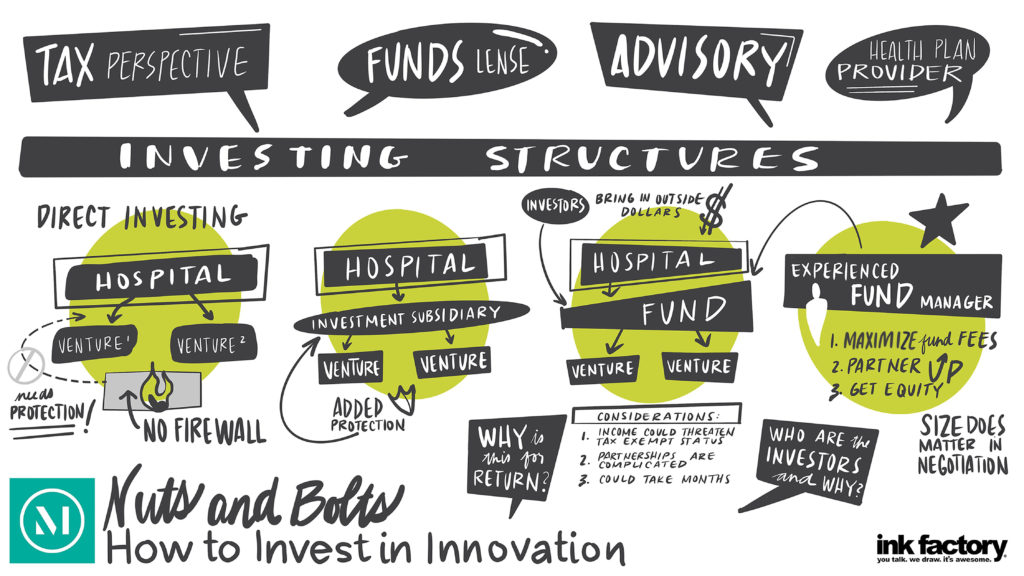 There has been increased interest by hospitals and health systems in creating innovation centers and making innovation center investments, which are helping to transform the healthcare landscape. As they enter this space, hospitals and health systems must first decide how to organize to capitalize on and commercialize innovation opportunities to get innovations into the routine of patient care.
There has been increased interest by hospitals and health systems in creating innovation centers and making innovation center investments, which are helping to transform the healthcare landscape. As they enter this space, hospitals and health systems must first decide how to organize to capitalize on and commercialize innovation opportunities to get innovations into the routine of patient care.




