In this session, panelists discussed recent technological, payment and regulatory trends in the ambulatory surgery center (ASC) space, with a focus on implementing and managing bundled payments. The group also explored complex questions raised on structuring and risk, leveraging technology to streamline ASC operations and maximize business outcomes, and structuring anesthesia arrangements for ASCs.
Session panelists included:
- Jason Scalise, MD, Chief Growth Officer, HOPCo
- Piper Su, Chief Growth Officer, triValence
- Tony Maida, Partner, McDermott Will & Emery
- Moderator: Ryan Higgins, Partner, McDermott Will & Emery
Top takeaways included:
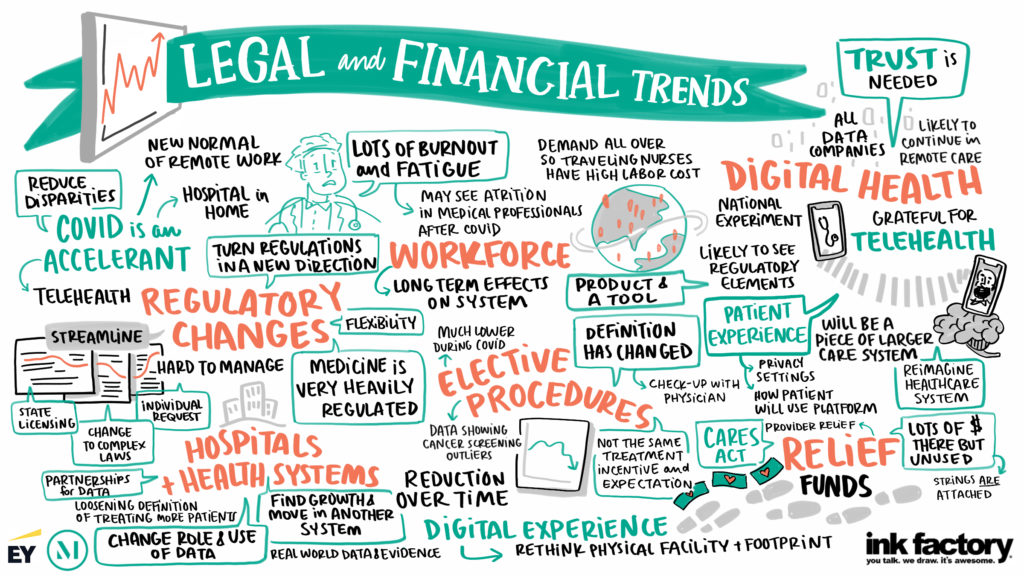
Bundled Payments
- There has been growing interest in expanding the time frame or scope of services included in a bundled payment. Such a shift in focus could help align behaviors and manage the appropriateness of care. But without appropriate real-time data, there are significant challenges in determining the scope and pricing of a bundle.
- As the scope of clinical services provided safely in an ASC has continued to expand, there has been a shift from inpatient to outpatient procedures, and driving them to ASCs. The ASC community can play an integral role in determining scope and pricing of bundles.
- Bundled payments have had a somewhat spotty reputation in terms of success. One challenge is that the payor may adjust the target annually, leading to cost-cutting and a race to the bottom until there is no opportunity for additional savings. Going forward, bundled payment programs should be developed with an eye toward sustainability, building in inflationary trends, demographic shifts and [...]
Continue Reading
read more

 Subscribe
Subscribe